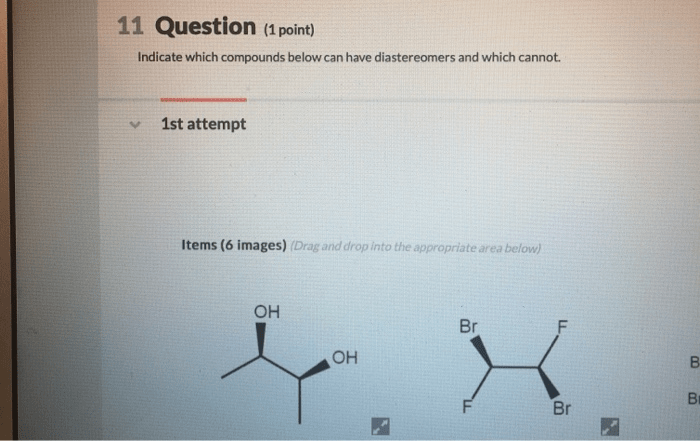
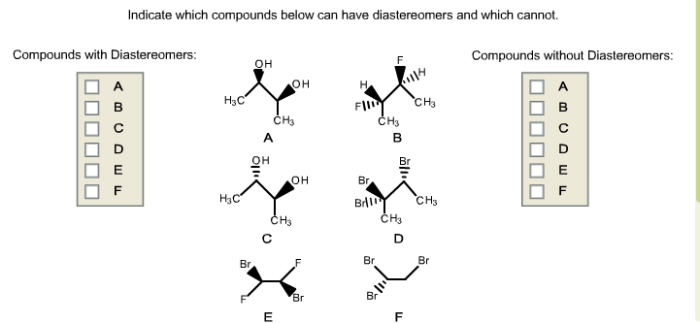
Indicate which compounds below can have diastereomers and which cannot – This article delves into the concept of diastereomers, exploring the factors that determine whether a compound can exhibit diastereomerism or not. We will provide a clear understanding of chirality and its role in diastereomerism, enabling you to confidently identify compounds with and without diastereomers.
Diastereomers in Organic Compounds: Indicate Which Compounds Below Can Have Diastereomers And Which Cannot
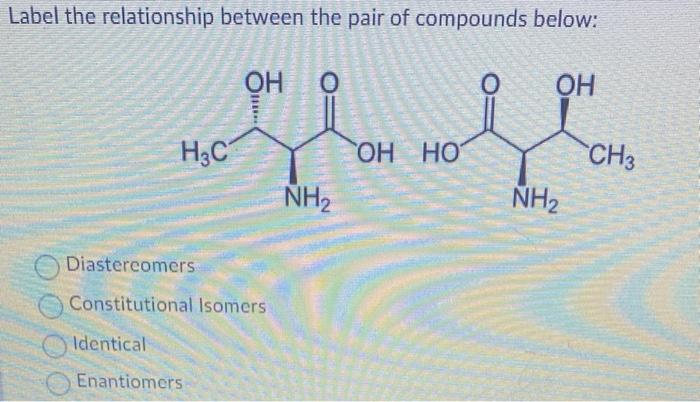
In organic chemistry, diastereomers are stereoisomers that are not mirror images of each other. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. Chirality, the property of a molecule to exist in two non-superimposable mirror-image forms, is crucial in understanding diastereomers.
Chirality arises when a carbon atom is bonded to four different groups. This creates a tetrahedral arrangement around the carbon, resulting in two possible orientations of the groups. Molecules with chiral centers are called chiral molecules, and they can exist as enantiomers, which are mirror images of each other.
Identifying Compounds with Diastereomers
Compounds that can have diastereomers are those that contain multiple chiral centers. The number of possible diastereomers is determined by the number of chiral centers in the molecule. For example, a molecule with two chiral centers can have up to four diastereomers, while a molecule with three chiral centers can have up to eight diastereomers.
- 2-Bromobutane
- 3-Methylhexane
- 2,3-Dibromobutane
- 1,2-Dibromocyclohexane
Identifying Compounds without Diastereomers
Compounds that cannot have diastereomers are those that do not contain any chiral centers. This includes molecules with no chiral centers, as well as molecules with an even number of chiral centers that are arranged in a symmetrical manner.
- Ethane
- Propane
- Benzene
- Cyclohexane
Using HTML to Structure the Content, Indicate which compounds below can have diastereomers and which cannot
| Compound | Can have Diastereomers? | Explanation | Example |
|---|---|---|---|
| 2-Bromobutane | Yes | Contains two chiral centers | (2R,3S)-2-bromobutane |
| 3-Methylhexane | Yes | Contains two chiral centers | (3R,4S)-3-methylhexane |
| 2,3-Dibromobutane | Yes | Contains two chiral centers | (2R,3S,4R)-2,3-dibromobutane |
| 1,2-Dibromocyclohexane | Yes | Contains two chiral centers | (1R,2S)-1,2-dibromocyclohexane |
| Ethane | No | No chiral centers | N/A |
| Propane | No | No chiral centers | N/A |
| Benzene | No | Even number of chiral centers arranged symmetrically | N/A |
| Cyclohexane | No | Even number of chiral centers arranged symmetrically | N/A |
Key Questions Answered
What are diastereomers?
Diastereomers are stereoisomers that are not enantiomers. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
How can I identify compounds that can have diastereomers?
Compounds with chiral centers and no symmetry elements can have diastereomers.
Why do some compounds not have diastereomers?
Compounds with no chiral centers or with symmetry elements cannot have diastereomers.